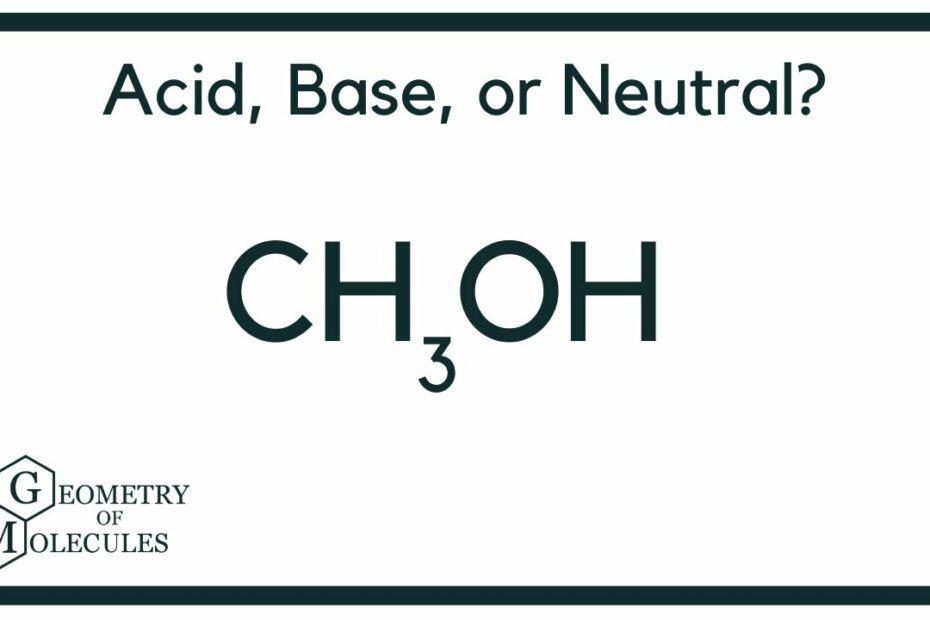
What Is The Ph Of Methanol: Unveiling Its Acidity Level
What Is The Ph Scale | Acids, Bases \U0026 Alkalis | Chemistry | Fuseschool
Keywords searched by users: What is the pH of methanol ph of methanol in water, ph of water, ph of methanol hplc grade, ph of ethanol, pH of methanol, ph of phenol, ph of dmso, acetic acid ph
Is Methanol Acidic Or Basic?
Is methanol acidic or basic? Methanol exhibits amphoteric behavior, meaning it can act as both an acid and a base. To determine its acidity or basicity, we can apply the Bronsted-Lowry theory, which categorizes substances as either proton donors (acids) or proton acceptors (bases). Methanol falls into the category of a proton donor because it readily donates a proton (H+) from its hydroxyl group (OH) when reacting with strong bases such as sodium hydride. This property allows methanol to exhibit acidic characteristics in certain chemical reactions while also retaining its role as a base when it acts as a proton acceptor in other contexts. Thus, methanol’s amphoteric nature makes it a versatile compound in various chemical reactions.
What Ph Does Methanol Have?
The pH of methanol is determined by the concentration of hydrogen ions (H+). In pure methanol, neutrality is achieved when the concentration of H+ ions equals that of CH3O ions, which happens at a pH of 8.3. When methanol is mixed with water, the autoprotolysis constants of the two substances come into play, ranging from 14 for water to 16.6 for methanol. As a result, the neutral pH range in these mixtures varies between pH 7 and pH 8.3. This means that methanol is more acidic than water and will require a higher pH level to reach neutrality when in a mixture with water.
Aggregate 6 What is the pH of methanol




Categories: Collect 89 What Is The Ph Of Methanol
See more here: chinhphucnang.com

Learn more about the topic What is the pH of methanol.
- pH in Methanol
- Methanol (CH3OH) – Structure, Molecular mass, Properties & Uses …
- The Interpretation of pH in Partially Aqueous Mobile Phases
- What is pH of methanol? – Quora
- Ethanol (C2H5OH) – JJS Technical Services
- Acid-Base – MSU chemistry
See more: blog https://chinhphucnang.com/dealbook