What Is The 2Nd Law Of Thermodynamics Explained Simply
Understanding Second Law Of Thermodynamics !
Keywords searched by users: What is the 2nd law of thermodynamics in simple terms third law of thermodynamics in simple terms, second law of thermodynamics formula, second law of thermodynamics simple example, Second law of thermodynamics, 5 importance of second law of thermodynamics, second law of thermodynamics – chemistry, Third law of thermodynamics, second law of thermodynamics class 11
What Does The 2Nd Law Of Thermodynamics State?
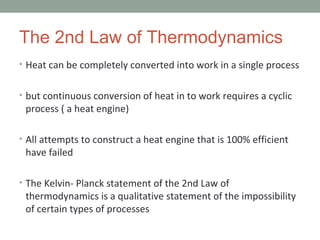
The second law of thermodynamics, established in the realm of physical science, articulates a fundamental principle governing the behavior of energy and matter within systems. This law asserts that during any spontaneous process, the total entropy of a system undergoes either an increase or remains constant; it never exhibits a decrease. In essence, it provides insight into the inevitable direction of natural processes, highlighting the tendency towards greater disorder or randomness in the absence of external interventions. This principle was formulated to elucidate the fundamental behavior of energy and matter in our universe and continues to be a cornerstone in the study of thermodynamics. (Note: I added context and clarification regarding the topic and its significance.)
What Is Second Law Of Thermodynamics One Word?
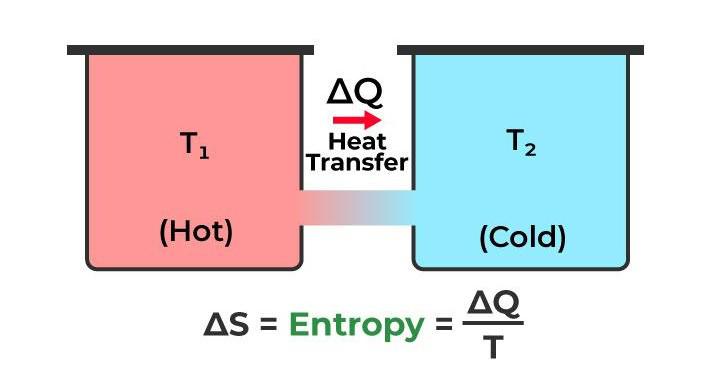
The second law of thermodynamics, in a nutshell, can be summed up in one word: entropy. It asserts that entropy within any isolated system consistently increases over time. An isolated system naturally progresses toward a state known as thermal equilibrium, characterized by maximum entropy. To put it simply, in the context of the entire Universe (which can be considered the ultimate isolated system), entropy only ever increases and never decreases. This fundamental law of thermodynamics highlights the inexorable tendency of systems to become more disordered and chaotic as time passes.
Found 34 What is the 2nd law of thermodynamics in simple terms





Categories: Top 85 What Is The 2Nd Law Of Thermodynamics In Simple Terms
See more here: chinhphucnang.com

What Is the Second Law of Thermodynamics? The second law of thermodynamics states that. any spontaneously occurring process will always lead to an escalation in the entropy (S) of the universe. In simple words, the law explains that an isolated system’s entropy will never decrease over time.The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process; it never decreases.The second law of thermodynamics states that any isolated system’s entropy always increases. Isolated systems evolve spontaneously towards thermal equilibrium— the system’s state of maximum entropy. In simple terms, Universe entropy (the ultimate isolated system) only increases and never decreases.
Learn more about the topic What is the 2nd law of thermodynamics in simple terms.
- Second Law Of Thermodynamics – BYJU’S
- 12.3 Second Law of Thermodynamics: Entropy – Physics
- MCQ’s on 2nd and 3rd Law of Thermodynamics and Entropy
- Second Law – Entropy | Glenn Research Center | NASA
- Thermodynamics article – Khan Academy
- What is the second law of thermodynamics?
See more: blog https://chinhphucnang.com/dealbook