Is Hno3 A Monoprotic Acid? Exploring Nitric Acids Proton Donating Properties
Monoprotic – Diprotic – Triprotic Substances: How Many Acidic Hydrogens Does The Substance Have?
Keywords searched by users: Is HNO3 a monoprotic acid Monoprotic acid là gì, Diprotic acid, pH of polyprotic acid, pKa of phosphoric acid, Describe what can be happened in the titration of a polyprotic acid H3PO4 by a strong base (NaOH)
Is Hno3 An Example Of A Monoprotic Acid?
Are Hydrochloric acid (HCl) and nitric acid (HNO3) monoprotic acids? Yes, they are. Monoprotic acids are substances that can donate only one hydrogen ion (H+) when they dissolve in water. Hydrochloric acid and nitric acid both fall into this category, even though they contain multiple hydrogen atoms in their molecular structure. Another example of a monoprotic acid is acetic acid (CH3COOH), which may seem to have more than one hydrogen atom but releases only one proton when it dissociates in water. So, in summary, HNO3 is indeed an example of a monoprotic acid, alongside HCl and CH3COOH. This clarification helps readers better understand the concept of monoprotic acids and why these specific chemicals are considered as such.
Is Hno3 A Strong Monoprotic Acid?
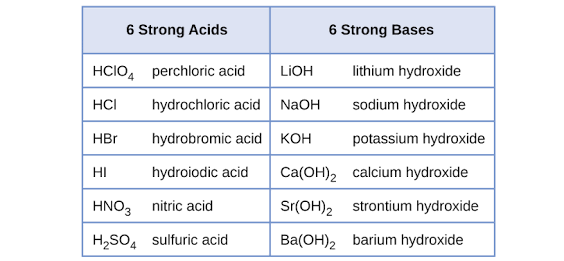
Is HNO3 considered a strong monoprotic acid? Nitric acid, denoted as HNO3, is indeed classified as a strong monoprotic acid due to its exceptional properties in aqueous solutions. When dissolved in water, nitric acid undergoes complete dissociation, breaking apart into its constituent ions. This means that every molecule of HNO3 separates into H+ ions and NO3- ions when dissolved in water, leaving no intact molecules. This behavior characterizes nitric acid as a strong acid. Moreover, it’s important to note that nitric acid yields precisely one mole of H+ ions for every mole of the acid itself. This distinct feature further solidifies its classification as a monoprotic acid. Thus, HNO3 stands as a well-known example of a strong monoprotic acid within the realm of mineral and inorganic acids.
Is Hno3 A Diprotic Acid?
Is HNO3 a diprotic acid? To answer this question, it’s important to understand the nature of nitric acid (HNO3). Nitric acid is classified as a monoprotic acid, indicating that each molecule of HNO3 can donate a single proton when it dissociates. In other words, in a chemical reaction, HNO3 releases one proton (H+) per molecule. To determine if it is diprotic, we need to explore whether it can donate two protons (H+) per molecule upon dissociation, which would be characteristic of a diprotic acid.
Details 37 Is HNO3 a monoprotic acid



:max_bytes(150000):strip_icc()/sulfuric-acid-molecule-147217372-5976342d5f9b5823a1d80e52.jpg)


Categories: Update 14 Is Hno3 A Monoprotic Acid
See more here: chinhphucnang.com

Hydrochloric acid (HCl), acetic acid (CH3CO2H or HOAc), nitric acid (HNO3), and benzoic acid (C6H5CO2H) are all monoprotic acids.Monoprotic Acid Examples
Hydrochloric acid (HCl) and nitric acid (HNO3) are common monoprotic acids. Although it contains more than one hydrogen atom, acetic acid (CH3COOH) is also a monoprotic acid as it dissociates to release only a single proton.Nitric acid is a strong acid that completely dissociates into its ions in aqueous solution. It is a common mineral/inorganic acid. Also, it produce one mole of H+ ions per mole of acid. Hence, it is a monoprotic acid.
Learn more about the topic Is HNO3 a monoprotic acid.
- Diprotic and Triprotic Acids and Bases
- Monoprotic Acid Definition – Chemistry – ThoughtCo
- Nitric acid is a: | Chemistry Questions – Toppr
- [Solved] Which one of the following is a diprotic acid A nitric …
- Monoprotic Acid Overview & Examples – Study.com
- Diprotic and Triprotic Acids and Bases
See more: blog https://chinhphucnang.com/dealbook